02/12/2026

Moderna Covid shots authorised for emergency use in Bangladesh
Staff Correspondent | Published: 2021-06-30 19:51:54
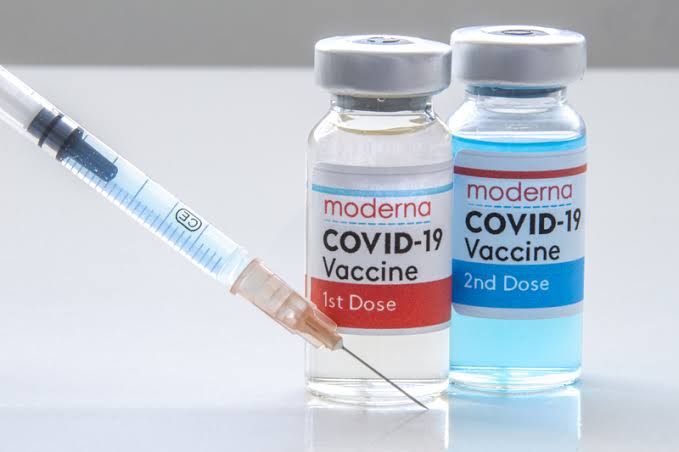
The Directorate General of Drug Administration on Tuesday night approved emergency use of Covid-19 vaccine Moderna in the country.
The regulator has given Emergency Use Authorisation (EUA) after all necessary examinations by the technical committee, a press release of DGDA said.
According to the DGDA, Moderna is the eighth vaccine to be given the EUA.
On June 15, DGDA approved emergency use of Johnson & Johnson's Covid-19 vaccine "Janssen" in Bangladesh.
On June 3, the Directorate General of Drug Administration (DGDA) approved the Chinese Covid-19 vaccine "CoronaVac", manufactured by Sinovac Life Sciences Company Limited.
On May 27, the DGDA approved emergency use of the Pfizer mRNA Covid-19 vaccine in the country.
Bangladesh has so far approved eight Covid-19 vaccines to control the deadly disease.
These vaccines are Covid-19 vaccine Moderna, Johnson & Johnson, "CoronaVac", manufactured by Sinovac Life Sciences Company Limited, Pfizer mRNA Covid-19 vaccine, Covishield produced by Serum Institute of India; Sputnik V manufactured by Generium Joint Stock Company of Russia, AstraZeneca manufactured by Sweden and Sinopharm produced by Beijing Institute of Biological Products Co Ltd of China.
Editor & Publisher : Md. Motiur Rahman
Pritam-Zaman Tower, Level 03, Suite No: 401/A, 37/2 Bir Protik Gazi Dastagir Road, Purana Palton, Dhaka-1000
Cell : (+88) 01706 666 716, (+88) 01711 145 898, Phone: +88 02-41051180-81