SAMI
Published:2020-10-03 20:26:02 BdST
Globe Biotech's 'BANCOVID' okay for human trial
Bangladeshi lone coronavirus vaccine candidate Globe Biotech has achieved another much-needed milestone, as a globally recognised journal has certified its 'BANCOVID' scientifically okay for human trial.
With the achievement, Globe Biotech overcame the last requirement in seeking ethical approval from Bangladesh Medical Research Council (BMRC) for conducting the vaccine's human modelling experiment.
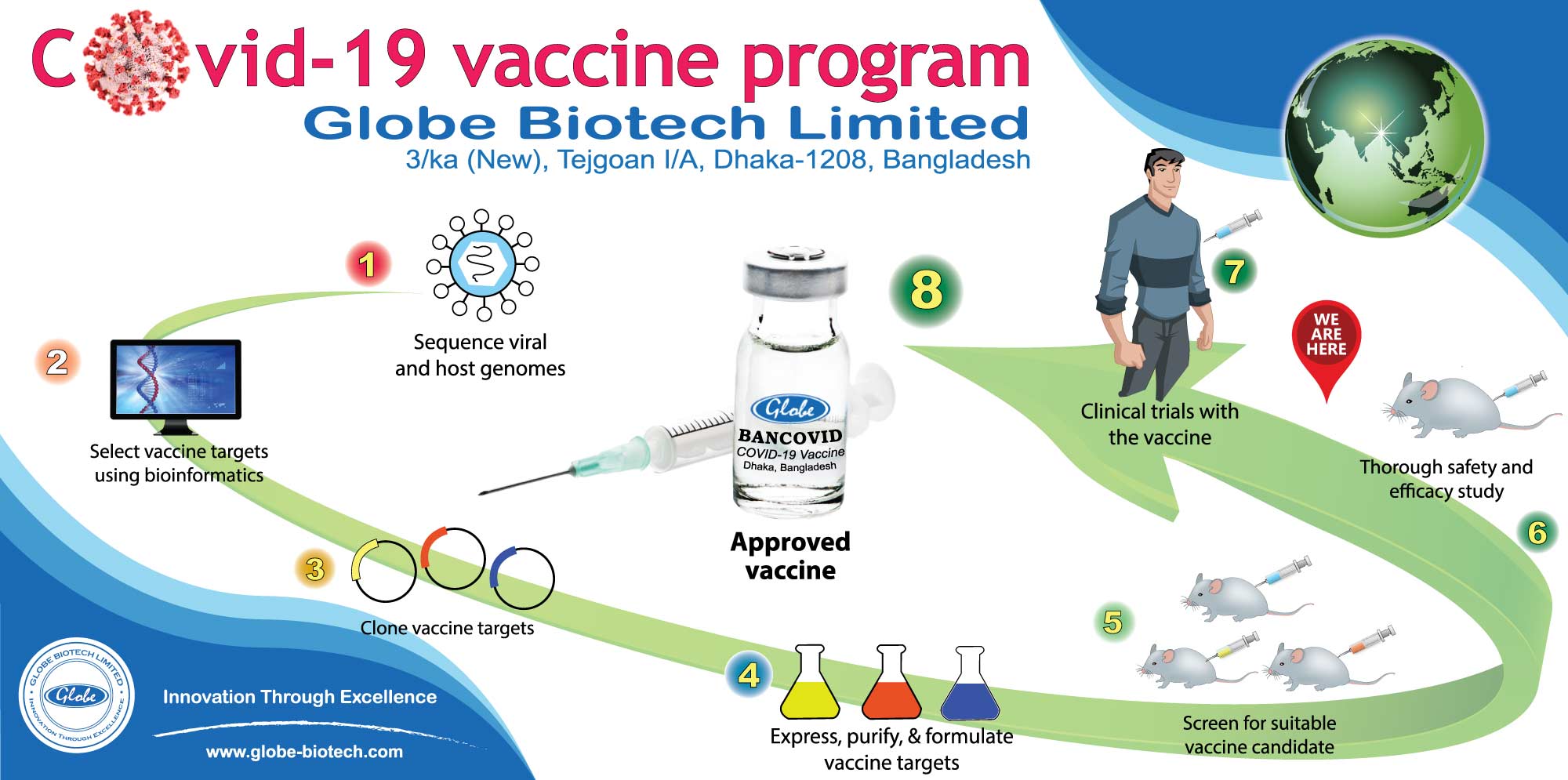
The New York-based Cold Spring Harbor Laboratory (CSHL)-operated bio archive or BioRxiv published the results of its pre-clinical trials on September 30.
It termed the Bangladeshi company's homegrown vaccine the first D614G variant mRNA-based vaccine candidate in the world that elicits neutralising antibody and balances cellular immune response.
Citing results of its animal modelling trials, CSHL stated that BANCOVID did not produce any noticeable effect for local or systemic toxicity as primarily evident by the absence of four cardinal signs of inflammation - redness (latin rubor), heat (calor), swelling (tumor) and pain (dolor).
The CBC (complete blood count) and blood chemistry did not show significant changes in relevant profiles, suggesting that the vaccine behaved safely in animals.
When contacted, manager (quality and regulatory operations) of Globe Biotech Dr Mohammad Mohiuddin said they did the coronavirus spike sequencing through which it enters into human body.
In the sequencing they found that the virus went through a genetic mutation in the position of its 614 number protein, which converted into G614 from D614, he also said.
Because of the transition, the virus became more infectious and deadly, and many global studies blamed the G614 variant for recent spread of the pandemic.
"Those who started developing vaccines in the early pandemic period did not consider the variant, but we did it. So, we are very confident that our vaccine will work, as it is scientifically proved."
Mr Mohiuddin added that they have brought the mutated deadly spike protein of the virus into their vaccine target sequencing to prevent spread of the infection.
Source at the company said it has planned to organise a press briefing on October 5 (Monday) to share the outcomes and possible date to launch the clinical trial before marketing the medicine commercially.
To start the first phase of human modelling trial for the much-talked vaccine, the local pharmaceutical company, through its CRO (contract research organisation), will officially seek ethical approval of BMRC within a few days.
The development came at a time when the government, like those of other states, is desperately looking for vaccines to contain the Covid-19 virus pandemic that has already spread throughout the country, killing at least 5,305 people till date.
Health and Family Welfare Minister Zahid Maleque said a high-level committee is communicating with the World Health Organisation (WHO) and possible vaccine manufacturers.
Another body, named the National Technical Committee, is helping them give necessary ideas and suggestions over vaccine-related developments.
Both the committees were told to communicate with the prospective vaccine producers, he further said.
"If any local company develops a prospective or quality vaccine, we will definitely consider it. We are monitoring Globe Biotech's development (in this regard)."
He noted that they are discussing with potential vaccine makers in China, the United Kingdom, India, France, the United States and Russia.
According to the global media, around 190 companies in the world are working on developing vaccines, including Moderna and Pfizer in the US, University of Oxford in the UK, Sinovac Biotech in China, Bharat Biotech in India, and Sanofi in France.
Of these vaccines, some nine are in phase-three stage.
Unauthorized use or reproduction of The Finance Today content for commercial purposes is strictly prohibited.